How to find moles? If you want to find moles, the best place to look is in your own backyard. Moles are small, burrowing animals that live underground. They are often found near areas of dense vegetation, such as forests or gardens. Moles can be a nuisance because they can damage lawns and gardens by digging holes.
However, they are also an important part of the ecosystem because they aerate the soil and help control pests. If you do find a mole in your yard, there are a few things you can do to get rid of it. You can trap it or use a Mole Repellent.
Definition of Number of Moles
In chemistry, a mole is a unit of measurement used to express the amount of a substance. One mole of a substance contains Avogadro’s number of molecules or atoms of that substance. The mole is also used to express the amount of a substance in terms of its elemental composition. For example, one mole of water (H₂O) contains two atoms of hydrogen and one atom of oxygen.
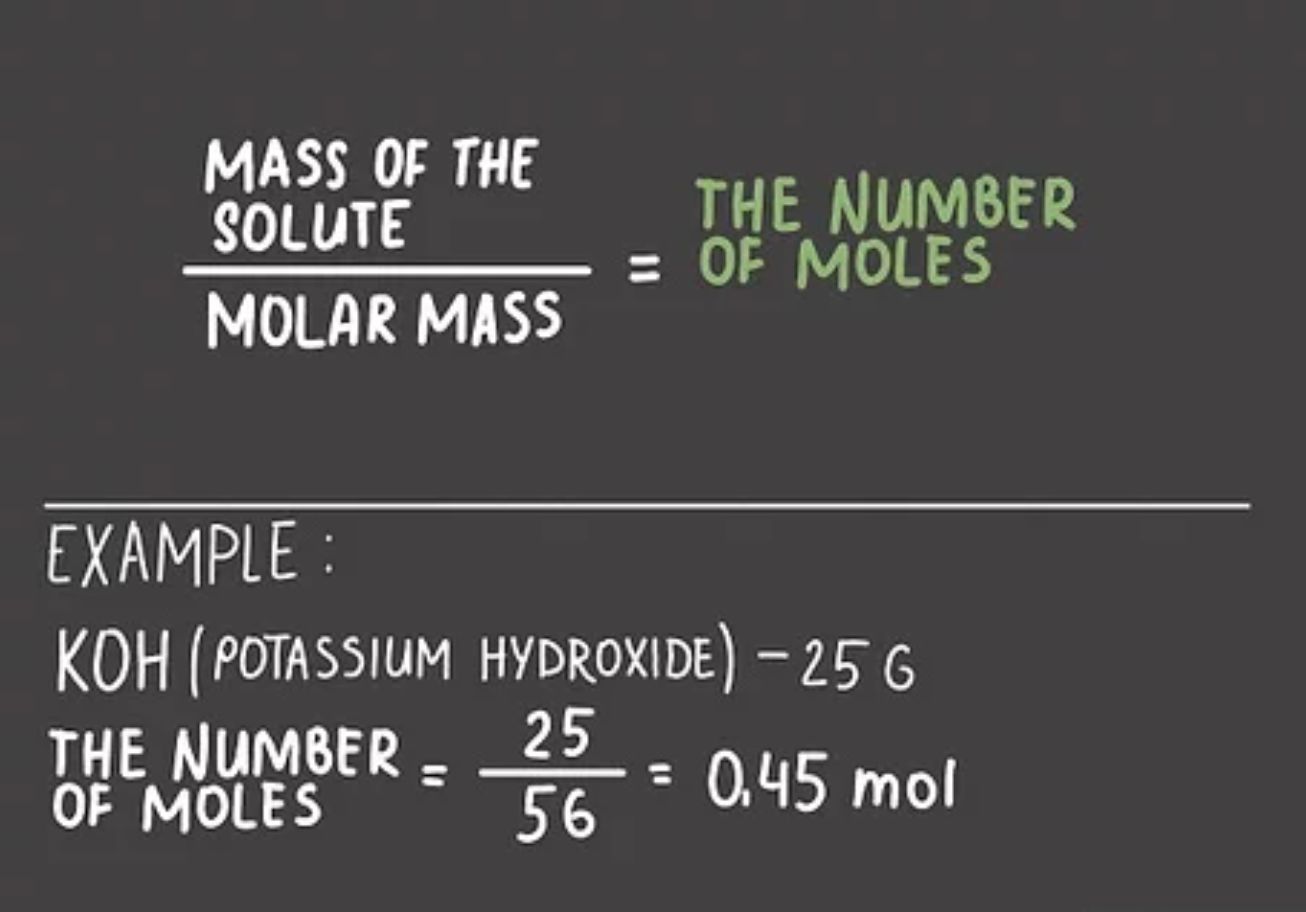
The number of moles (abbreviated as “mol”) in a sample can be determined by using the formula:
Number of moles = Mass ÷ Molar mass
Where:
mass is the mass of the sample in grams; and
molar mass is the molar mass of the substance in grams per mole.
How to Find Moles?
Moles can be found in a number of ways. The most common method is to use a mole trap. This is placed in an area where moles are active and will kill the animal when it enters the trap. Other methods include using poison or gas to kill the animals.
Another way to find moles is to look for their tunnels. These can be found by looking for raised dirt hills in your yard. If you see one of these, it’s likely that a mole has been burrowing underneath. You can also try to flush the animal out by pouring water into the tunnel.
Molarity Calculation
In order to calculate molarity, one must first determine the number of moles of solute present in the solution. This can be done by using the molar mass of the solute and dividing it by the mass of the solution. Once the number of moles is known, the molarity can be calculated by dividing it by the volume of the solution.
Molarity is a unit of concentration that tells us how many moles of a substance are present in one liter (L) of solution. To calculate molarity, we need to know two things: 1) how many moles of solute are present in our solution and 2) the volume of our solution. Once we have that information, we simply divide the number of moles by the volume to get our answer.
Determine the Molecular Mass of a Chemical Compound
When trying to determine the molecular mass of a chemical compound, there are a few different ways that you can go about it. The first way is to look at the periodic table and find the atomic masses of each element that makes up the compound. Once you have those numbers, you simply add them all together to get the molecular mass.
Another way to determine the molecular mass of a chemical compound is by using a mass spectrometer. This instrument can be used to measure the masses of different molecules and atoms. By doing this, you can more accurately determine the molecular mass of a chemical compound.
The last way to determine the molecular mass of a chemical compound is by using its formula. This information can be found on the label of most chemicals.
Determining the Molar Mass of an Element
In order to determine the molar mass of an element, one must first know the atomic mass of the element in question. The atomic mass can be found on the periodic table of elements.
Once the atomic mass is known, the molar mass can be determined by using the following equation: Molar Mass = Atomic Mass/Molar Mass Constant. The molar mass constant is equal to Avogadro’s number, which is 6.022×10^23 atoms/gram. This number can also be written as a mole.
Therefore, one mole of any element will have a molar mass equal to its atomic mass in grams. For example, if we want to determine the molar mass of oxygen, we would find that its atomic mass is 16 grams/mole. This means that one mole of oxygen has a molar mass of 16 grams.
Converting From Mass to Moles
To convert from mass to moles, you need to know the molar mass of the substance. The molar mass is the number of grams in one mole of a substance. To find the molar mass, you can look it up in a periodic table or use a calculator. Once you have the molar mass, you can divide the mass of the substance by the molar mass to find the number of moles.
For example, suppose you have 12 grams of sodium chloride (NaCl). The molar mass of NaCl is 58.44 grams/mole. So, to convert from grams to moles, you would divide 12 by 58.44 to get 0.205 moles of NaCl