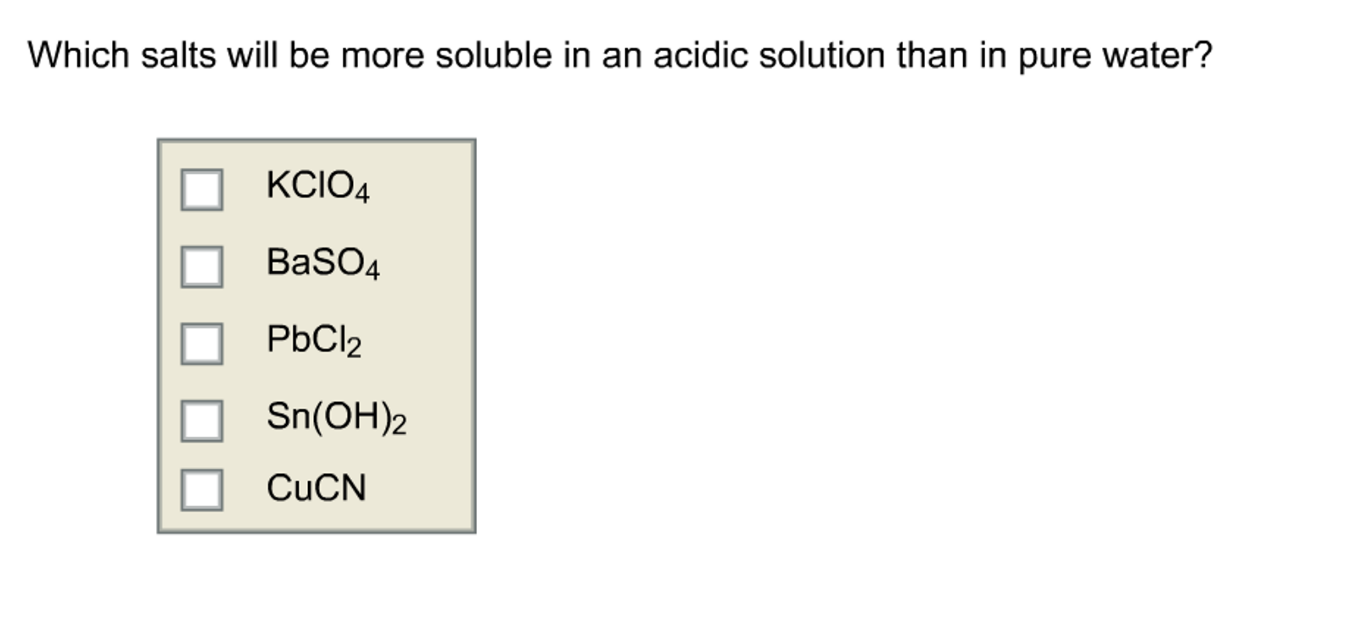
Which of the following salts will be more soluble in an acidic solution than in pure water?

Neutralization Reaction:
When an acid (HA) and a base (BOH) react, they generally form a salt (BA) and water as shown in the general equation below:
HA+BOH→BA+H2O
The salt product can be classified as either acidic, basic, or neutral depending on the strength of the acid and base that formed it.
the anion of salt is able to react with an H+ ion to form a weak acid, it can be removed from the solution by adding an acid.
For AgI adding H+ forms HI and that is a strong acid; therefore, AgI is NOT more soluble in acid than in water.
For Sn(OH)2, adding H+ reacts with OH- to form H2O so YES, it is more soluble in acid than in H2O
For KClO4, adding H+ forms HClO4 but that is a strong acid so it is NOT more soluble in acid.
For CuBr adding H+ forms HBr and that is a weak acid; therefore, CuBr is more soluble in acid than in water.
For Ag2SO4 adding H+ forms HSO4- and that is k2 for H2SO4, there is no k1 since H2SO4 is a strong acid for the first H. so YES more soluble in acid than in water.
For BaSO3 adding H+ forms HSO3- and that is k2 for H2SO3, there is no k1 since H2SO3 is a strong acid for the first H. so YES more soluble in acid than in water
SrSO4 would be HSO4^-
CaCO3 would be HCO3^- which is k2 for H2CO3 and YES.